Catalysing cancer research: the value of transparent clinical trial data

Clinical trial transparency is essential for advancing cancer research and improving treatments
Advancements in the quest for improved cancer treatments depend strongly on streamlined and efficient research, made possible by the accessibility of clinical trial data from research conducted globally.
Clinical trial transparency refers to the practice of making all relevant information about a clinical trial publicly available. This includes details about the study design, methodology, results, and any adverse events that occurred during the trial.
Transparent clinical trials allow researchers, healthcare professionals, policymakers, and the public to access accurate and complete information about the research process and its outcomes. This transparency promotes scientific integrity, reproducibility of results, and helps healthcare providers and patients to make informed decisions about care and treatment.
Public disclosure of clinical trial information and documents is required in countries and regions around the world, based on a regulatory framework. Among these, the European Union (EU), Canada, Japan, and the United States (US) are notable for having some of the most comprehensive regulations regarding this topic.
But this has not always been the case.
Historically, transparency in clinical trial results was severely lacking
For a long time, transparency regarding clinical trial results was severely lacking. Merely half of the conducted trials were published, with a notable bias toward publishing those with positive outcomes over negative ones. This imbalance resulted in unnecessary duplication of studies and ill-informed decisions regarding the treatment of cancer patients.
In 2014, there was a significant development in clinical trial transparency with the implementation of new regulations in the EU, when it adopted the Clinical Trials Regulation (EU) No 536/2014, which aimed to improve transparency and accessibility of clinical trial data. This regulation established requirements for the registration and public disclosure of clinical trial information, like study protocols and summary of results. It also introduced measures to ensure the timely reporting of trial results within the EU Clinical Trials Register (EUCTR), which replaces the former EudraCT registry. This development represented a crucial step forward in ensuring transparency in clinical research and establishing a benchmark for other regions to follow.
Despite regulations, gaps in transparency persist
Despite these developments, several gaps in clinical trial transparency have persisted, including selective, delayed, or incomplete reporting, as well as inaccessibility of data. A group of researchers has been tracking, first in 2018, and then in 2023, whether clinical trial results were being reported to the EU Clinical Trials Register within a year after the study finished, as required by the European Commission. They saw modest improvement between 2018 and 2023. In their 2023 report, they compared the availability of trial results on the EU Clinical Trials Register with other methods of sharing results. They found that the availability of results on the EU Clinical Trials Register was similar to that of scientific journals and greater than on other registries like ClinicalTrials.gov, the US registry.
Interestingly, both studies showed that trials that had a commercial sponsor were more inclined to report their results compared to trials without a commercial sponsor (see graph below). (Goldacre B, et al. BMJ 2018;362:k3218 and De Vito NJ, et al. BMJ 2024;3:e000783)
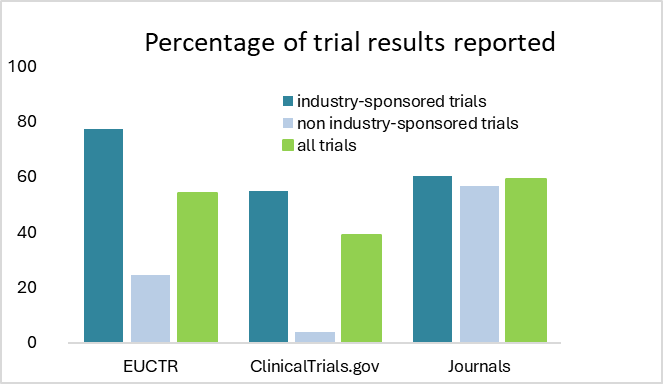
Based on data from DeVito et al. 2023
Overall, there is room for improvement. Regulators as well as trial funders can play a particularly significant role in improving the situation.
The EU revised its transparency policy, implementing clearer guidelines
The EU Portal and the EU Clinical Trials Register, for the exchange of information on clinical trials in the European Union, have been in use since 31 January 2022. The EU Portal is the online platform where sponsors submit, and regulatory authorities review applications for clinical trials conducted in the EU. It is part of the Clinical Trials Information System (CTIS), the ‘central control centre’ where researchers, regulators, and other people involved in clinical trials can go to handle all the paperwork and information related to those trials.
Originally, the Clinical Trials Information System (CTIS) allowed for uploading two versions of a document: one marked "for publication" and the other "not for publication," aimed at protecting personal data in the submitted documents. However, it has become clear that the "not for publication" option has been used not just for personal data protection, but also to safeguard commercially confidential information.
The protection of commercially confidential information was also facilitated through a delay mechanism, which offered different options based on the categorization of the trials, recognizing that clinical trials vary in the types of commercially confidential information they contain, particularly depending on the stage of development of the investigational medicinal product.
This trial categorization determined the applicable deferral timelines, allowing sponsors to publish certain clinical trial information not immediately upon the application decision. Instead, it could be delayed for up to 7 years after the trial's completion.
The European Medicines Agency (EMA) conducted a public consultation between May and June 2023 regarding the revision of the transparency regulations. The feedback from the consultation, which received over 200 responses, highlighted diverse views on the use of deferrals and redaction functionalities, with a consensus on the need for simplification.
After this public consultation, the EMA updated the CTIS transparency rules that included more than just removing the deferral timelines. They set up a system that categorizes trials into different types and specifies the timelines of when certain information, like study protocols, summaries, and results should be shared based on the type of trial and whether it involves adults or children. This makes it clearer when and what information about trials should be available to the public.
So, from now on, the study protocols for most clinical trials will be publicly available on the new European CTIS trial registry (EU Clinical Trials Register) immediately upon regulatory approval for the trial, whereas before, pharmaceutical companies could postpone the publication of study protocols for several years following the completion of the trial.
The Anticancer Fund is actively involved in improving reporting standards
The Anticancer Fund remains committed to promoting clinical trial transparency through various efforts.
One significant challenge in clinical trial reporting is that transparency does not offer immediate benefits to individual investigators. Instead, its benefits lie in serving the broader interests of the research community, and ultimately the patients. Increasing awareness among researchers and sponsors about these benefits is a key goal for us.
As a member of ICRP (the International Cancer Research Partnership) and a founding member of the Belgian Cancer Research Consortium, that focusses on improving clinical trial reporting for trials funded by Belgian funding organizations, we are actively involved in enhancing clinical trial reporting. Additionally, we enforce our own clinical trial transparency policy on the trials we fund.
Looking ahead, the Anticancer Fund is continuously looking to improve clinical trial reporting, especially for the trials that are supported by us. One of the things we are considering next, is introducing an additional funding milestone for trials that fulfil all reporting requirements.
